SCDC Update
ASH’s 60th Annual Meeting Recap
The American Society of Hematology’s (ASH) 60th Annual Meeting and Exposition took place December 1-4, 2018, in San Diego, California. The meeting provided an invaluable educational experience and the opportunity to review thousands of scientific abstracts, highlighting updates and exciting developments in the hottest topics in hematology, including sickle cell disease (SCD). In addition to sessions and abstracts, this year’s meeting featured an SCD Kiosk where attendees could preview ASH’s SCD curriculum for fellows, learn about the overall initiative, hear five minute rapid fire SCD presentations, and play Jeopardy where attendees were quizzed on their knowledge about the state of SCD in 2018. For an SCD roundup at the ASH Meeting, click here. For those who missed a session or two, they are now available for purchase on ASH on Demand.

Applications to Join ASH Research Collaborative SCD Clinical Trials Network Due January 31
The Sickle Cell Disease (SCD) Clinical Trials Network (CTN) is an ASH Research Collaborative initiative launched with a mission of improving outcomes for individuals with SCD by expediting SCD therapy development and facilitating innovation in clinical trial research. It will provide the infrastructure for identifying patient cohorts for trials, matching trial sponsors with sites, facilitating recruitment of eligible patients, and ensuring optimally designed trials and an efficient, coordinated approach. Through patient engagement and optimized clinical trial execution, the Network will help bring new and more effective therapies to individuals with SCD. To apply to become a Network site, submit a letter of intent by January 31, 2019 to SCD-CTN@ashresearchcollaborative.org. Invited applicants will have full proposals due Spring 2019. Selected sites will then undergo on-site evaluations. For more information and questions regarding the Network, please view the FAQs or contact ASH RC CTN Staff at SCD-CTN@ashresearchcollaborative.org.
NHLBI’s Cure Sickle Cell Initiative Offers Services for Researchers
The Cure Sickle Cell Initiative (Initiative) was created by the National Institute of Health (NIH)’s National Heart, Lung, and Blood Institute to accelerate the development of treatments aimed at a genetic-based cure for sickle cell disease to improve the lives of its patients. Amongst the services that the Initiative provides is assistance to the researcher community with navigating the regulatory pathway and analytical support during the development process of their investigational therapy. In that regard, the Initiative collaborates with the Production Assistance for Cellular Therapies (PACT) program, an NIH wide resource, to provide regulatory assistance to investigators who may require support with guidance for pre-pre-IND (INTERACT) and pre-IND discussions the with the Food and Drug Administration (FDA), pre-clinical study design, Chemistry, Manufacturing, and Controls (CMC) development, as well as initial Investigational New Drug (IND) preparation. Additionally, investigators may request support in the form of a Gap Analysis which may cover areas such as manufacturing processes for both nonclinical and clinical trial materials, process validation, development and scale up, standard operating procedure development and batch production records, product cryopreservation, technology transfer, IND submission assistance, phase I clinical development plan, as well as FDA communications and commitments. For further information, please visit: curesickle.org.
New Study Finds Hydroxyurea Safe for Children in Africa
A new study published in the New England Journal of Medicine that followed 600 children in Angola, Uganda, Kenya and the Democratic Republic of Congo as they took hydroxyurea, found that not only did it ease painful crises, it reduced the risk of malaria infection as well. Although more research remains to be done, knowing that hydroxyurea, a cheap, effective and easy-to-take pill can safely be given to African children, may save millions of youth from agonizing pain and early deaths. Read more about it in a ‘From Nothing to Gangbusters’: A Treatment for Sickle-Cell Disease Proves Effective in Africa, a New York Times article, which includes an interview from Dr. Ify Osunkwo, representative from the Sickle Cell Adult Provider network, a member organization of the Sickle Cell Disease Coalition. 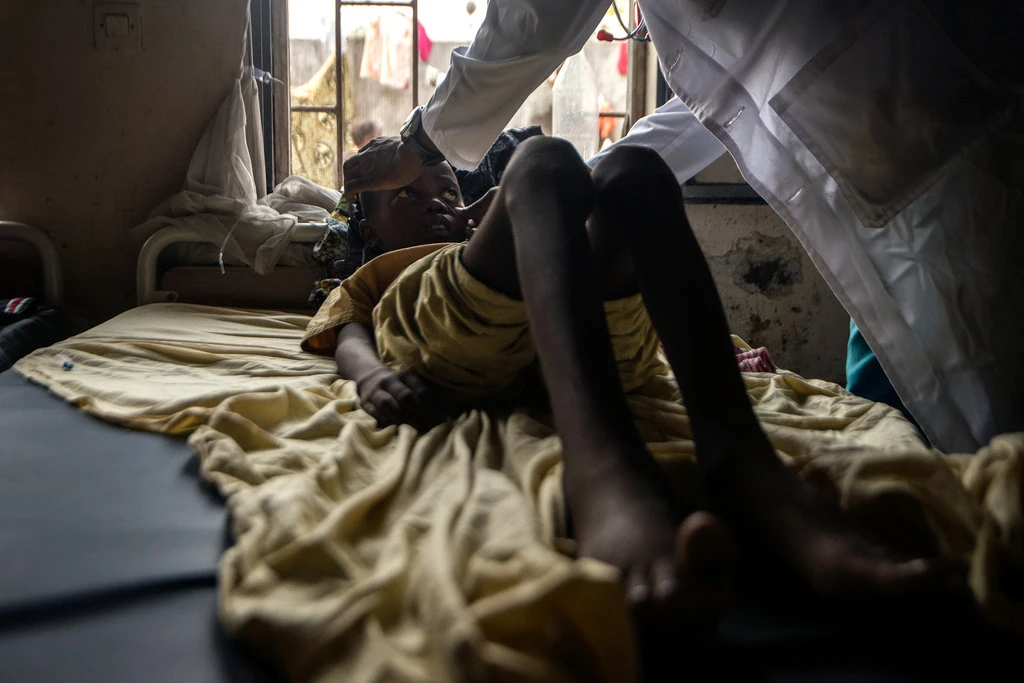
Sickle Cell Disease Bill Passes in Congress and Heads to the President for Signature
On Tuesday, December 11, the Sickle Cell Disease and Other Heritable Blood Disorders Research, Surveillance, Prevention, and Treatment Act (S. 2465) sponsored by Senators Tim Scott (R-SC) and Cory Booker (D-NJ), was approved by the House of Representatives. Having already passed in the Senate, the legislation is now waiting to be signed into law by the President. This important legislation reauthorizes sickle cell disease (SCD) prevention and treatment grants awarded by the Health Resources and Services Administration (HRSA) and authorizes the federal government to award data collection grants to states, academic institutions, and non-profit organizations with the goal of better understanding the prevalence and distribution of heritable blood disorders, including SCD, and the associated health outcomes and complications of these disorders. Congratulations to the many SCD advocates who helped lead to this victory.

2018 ASH Advocacy Efforts Related to Sickle Cell Disease and Sickle Cell Trait
The American Society of Hematology (ASH) continues to work with federal agencies and the U.S. Congress to help enhance and expand government activities in sickle cell disease (SCD) research, training, and services. ASH is working with congressional champions to raise awareness for SCD on Capitol Hill. ASH and the Society’s advocates took a number of steps in 2018 to advance the Society’s SCD-focused advocacy agenda. For more information, click here.
New! CDC Resources
Check out and share the Center for Disease Control and Prevention (CDC)’s new sickle cell disease resources and information about an upcoming webinar:
- New publication: Effectiveness of Clinical Decision Support Based Intervention in the Improvement of Care for Adult Sickle Cell Disease Patients in Primary Care
- Blood donation animation: Learn about minority blood donations from a new animated graphic! Share it on social media to help spread awareness about African-American blood donations.
- Sickle Cell Trait (SCT) Toolkit Now in French and Spanish! The SCT Toolkit includes factsheets and infographics on various health topics related to SCT. Visit the SCT Toolkit webpage to access the English, Spanish, and French versions of these materials.
- “The Bloodline” newsletter archive: “The Bloodline” is a quarterly newsletter that provides updates about the Sickle Cell Data Collection (SCDC) program. Subscribe to the newsletter and access past issues here!
- Don’t miss an upcoming SCDC webinar: The SCDC program’s California team will be hosting “Community Health Workers and Mobile Apps for Transition from Pediatric to Adult-focused Sickle Cell Care” on December 13th. Join the email list to receive announcements about upcoming webinars.
New One-Stop Webpage for SCD Education & Resources
The National Donor Program/Be The Match has created a new webpage to find educational activities on the latest sickle cell disease (SCD) treatment options, clinical trials for SCD, referral guidelines, and resource materials for clinicians, patients and families.
Discussing SCD therapy options with patients and caregivers is an ongoing dialogue throughout the course of treatment. The educational activities and resources, provide strategies to engage and educate patients and families with SCD, so that they can make an informed treatment decision.
On the page, you will find:
- Links to three live or on demand SCD educational activities in multiple formats.
- Information on current Blood and Marrow Transplant Clinical Trials Network trials for transplant and SCD.
- A link to the Jason Carter Clinical Trials website that can make it easier for you and your patients to find and join clinical trials for SCD and other blood disorders.
- Links to several clinical decisions making resources for physicians and patients including disease fact sheets.
Upcoming SCD Events
Save the DATE-FSCDR Symposium
The Foundation for Sickle Cell Disease Research will be hosting its 13th Annual Symposium June 7-9, 2019 in Fort Lauderdale, FL. Registration is now open.
Save the DATE-SCDAA Convention
Sickle Cell Disease Association of America’s 47th National Convention will be held October 9-12, 2019 in Baltimore, MD.
Spread the Word
The status quo is unacceptable & we are setting out to change it. Join us to #ConquerSCD! www.scdcoalition.org